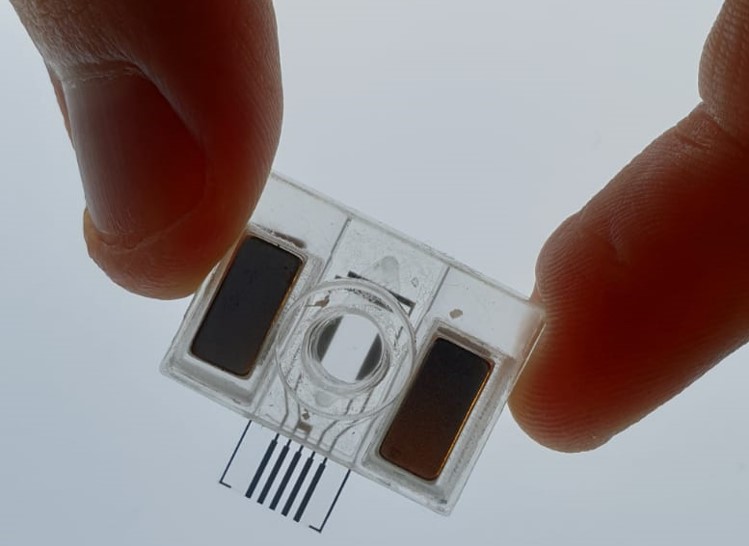
Technological developments for New Approach Methods (NAMs) in Environmental Risk Assessment
The recent New Approaches Methodology (NAMs) Network launch event [1] at the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) offices in London and the SETAC (European Society for Environmental Toxicology and Chemistry) Europe 34th Annual Meeting [2] in Sevilla provided an ideal opportunity to reflect on alternative applications of the technologies being developed within the AquaBioSens project.
For those not familiar with NAMs: It refers to the development of new computer-based or in vitro technologies for environmental risk assessment, with ecoNAMs being an off shoot applied to ecological risk assessment.
Historically for human risk assessment, we have used mammalian (rats, mice) toxicity tests to derive safe limits of chemical exposure. This may have been the best approach at the time, but to state the obvious, rodents are not humans and have different physiological responses to chemicals. The experimentation on animals is also ethically questionable, especially if the data generated does not necessarily address the issue it was intended to solve. Society is aware and legislation has banned the use of animal tests in the cosmetic industry and around the globe. Additionally, there is a move towards reducing the number of animals used in chemical risk assessment. In most countries there is an equivalent to the UK’s NC3Rs, dedicated to funding research to develop acceptable replacement for animal toxicity tests.
NAMs are built on another concept termed the Adverse Outcome Pathway (AOP) which describes the cellular processes that lead to an adverse outcome. A well-established AOP is oestrogen receptor activation where an oestrogenic compound binds to the receptor – the molecular initiating event (the MIE). The MIE causes expression of the gene vitellogenin in oviparous organisms – a key event (KE) – which is occurring in a male oviparous organism and leads to intersex – an adverse outcome (AO).
The molecular initiating event and key events are the two processes where it is possible to replace whole organism testing with cell based microfluidic biosensing devices. For example, high-throughput cell-based assays can be used to assess the binding of chemicals to human proteins and the ToxCast/Tox21 initiative [3] in the United States uses a robotic platform to screen 10,000s chemicals. This information can then rank the chemicals in terms of their hazard. To evaluate risk, however, we need more information on the toxicokinetic (TK), how the body metabolises and eliminates the compound, and toxicodynamic (TD), the effect of a chemical on the body. It is the TKTD process that offers the best opportunity to develop novel NAMs technologies making use of organoid culture techniques, co-culture microfluidic devices, high-throughput sequencing and mass spectrometry and simpler ways to report on adverse outcome endpoints.
It will be exciting to see how some of the technological advances made in AquaBioSens for environmental biosensing can be transferred to the growing NAMs market.
Author: Dr Nic Bury
Links
[1] NC3Rs, ‘NAMs Network’, NC3Rs. Available: https://nc3rs.org.uk/3rs-resource-library/nams-network. [Accessed: Jun. 10, 2024]
[2] SETAC, ‘SETAC Europe 34th Annual Meeting’, SETAC. Available: https://www.setac.org/discover-events/global-meetings/setac-europe-34th-annual-meeting.html. [Accessed: Jun. 10, 2024]
[3] US EPA, ‘Toxicology Testing in the 21st Century (Tox21)’, EPA United States Environmental Protection Agency, Jun. 03, 2024. Available: https://www.epa.gov/chemical-research/toxicology-testing-21st-century-tox21. [Accessed: Jun. 10, 2024]
Figure
Picture kindly provided by Professor Hywel Morgan, University of Southampton.
Keywords
New Approaches Methodologies, NC3Rs, molecular initiating events, key events, adverse outcome pathways, AOP, high throughput screening, microfluidics, organoids